H3ABioNet provides guidelines on the use of clinical data, data management and data standardization
Data & Standards
Data Standardisation
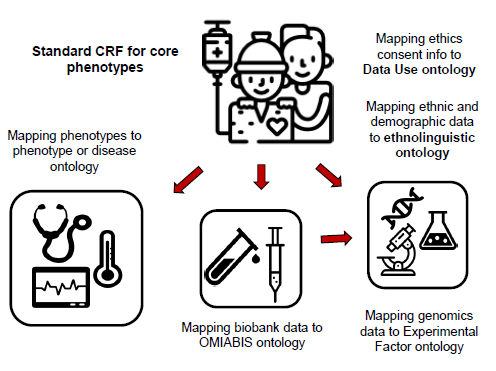
Standard CRF
H3ABioNet has worked with the Phenotype Harmonization Working Group of H3Africa to establish data collection tools for a recommended set of phenotype data elements. This has been dubbed the “Std. CRF”.
The acronym ‘CRF’ stands for Case Report Form and these are forms (hardcopy or electronic) that are used to collected research data from study participant for the study database.
Data collected with these forms provides the phenotype data complementing the genomic data generated from DNA samples from the H3Africa studies participants.
H3ABioNet has built resources for the Std. CRF in the form of a REDCap template (this offers a REDCap xml file which can be immediately uploaded in the REDCap platform as a complete project incorporating a Data Dictionary with the standardised data definitions and formats for H3Africa recommended phenotypes for collection.
A Std. CRF Guidelines document describes the H3Africa Std. CRF and provides the full set of CRFs with instructions and guidance on each data element.
- Download the Std. CRF Guidelines document Additional work is currently underway to establish a set of paediatric phenotype data elements recommended for standardised data collection.
Pdf files of Case Report Forms collecting the recommended variables were generated using Microsoft Publisher which is included in the Office suite and as Data Managers generally would take these variables and add in their own study required variables, editable MS Publisher files of each CRF are also available for adaption in this zipped file (WARNING: Caution is advises if rearranging the order of questions on the forms – ensure the flow of data collection and skip patterns does not get disrupted)
OR, select individual pdf forms from the Std. CRF
- Demographics
- Smoking Status
- Alcohol Consumption
- Drug Use
- Anthropometrics
- Blood Pressure
- Urine Test Results
- Kidney Disease
- Prescribed Medications
- CVD
- Stroke History
- Diabetes
- HIV
- Dyslipidemia
- Cancer
- Infectious Diseases
Additional work is currently underway to establish a recommended set of standardised paediatric phenotype data elements to include in the Std. CRF.
Domain-Specific Minimum Data Reporting Guidelines
Domain-specific studies can be poorly articulated and indexed, raising the need for minimum reporting requirements and (or) guidelines to fully understand the context, methods, data and conclusions that pertain to a study and/or experiment. The primary aim of a guideline is to provide broad guidance for a specific study’s data collection and management.
Based on the Phenotype Standards captured within the Standard CRF, H3ABioNet is developing domain-specific minimum reporting guidelines, subdivided into three sections (participant-; study-; and experiment-specific information) which can be employed to promote comprehensive and harmonized data collection within specific domains. These domains include:
- Kidney Disease
- Stroke
H3ABioNet has built resources for the reporting guidelines in the form of a XML schemas, which can be uploaded on a data management platform such as REDCap.
- Download the XML schemas for minimum data reporting guideline for KIDNEY DISEASE research.
- Download the XML schemas for minimum data reporting guideline for STROKE research.
Associated data dictionaries have been completed for each reporting guideline and are available in .csv format. These data dictionaries outline ontologies to which each data element is mapped to.
- Download the data dictionary (.csv) for KIDNEY DISEASE research.
- Download the data dictionary (.csv) for STROKE research.
A Domain-Specific Minimum Information Data Reporting Guidelines: Recommendations for Use document has been created in order to guide users in the employment of these reporting guidelines and data dictionaries.
- Download the Domain-Specific Minimum Information Data Reporting Guidelines: Recommendations for Use
Work is ongoing to extend domain-specific data reporting guidelines where the needs arise.